 Jan, 29 2026
Jan, 29 2026
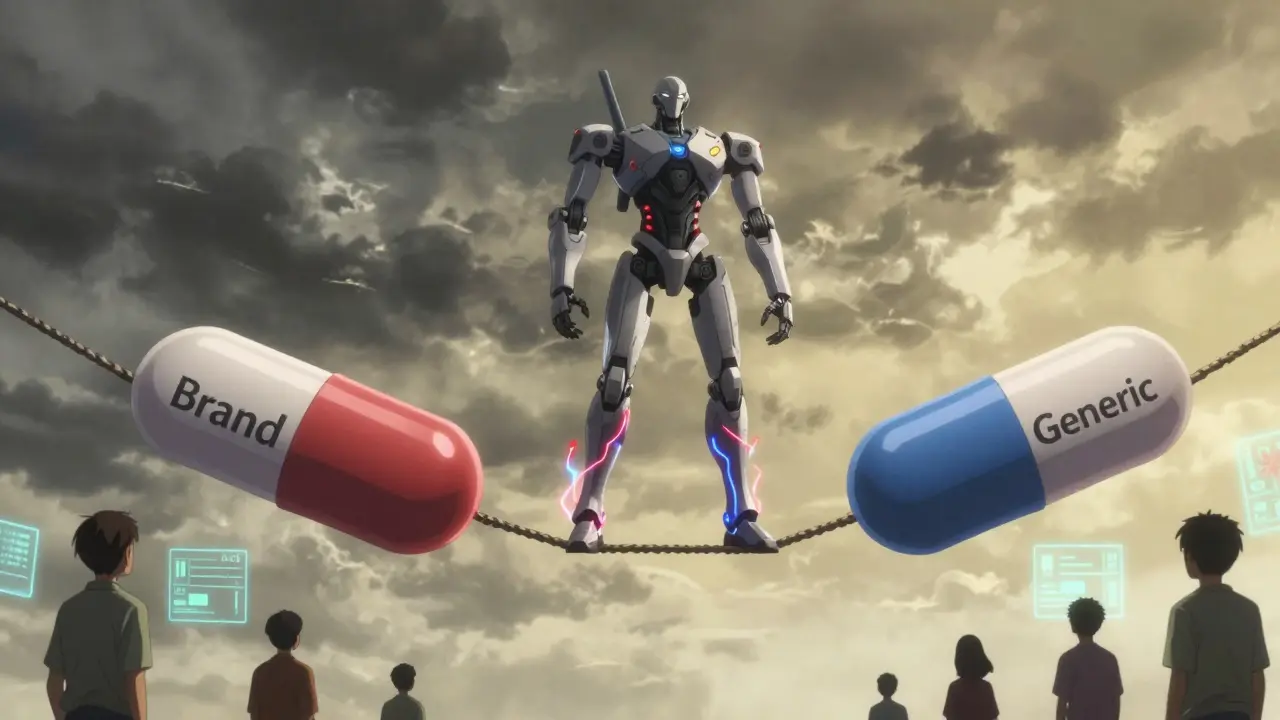
When a drug has a narrow therapeutic index (NTI), even tiny differences in how it’s absorbed or processed by the body can mean the difference between healing and harm. Think of it like walking a tightrope - one small misstep, and you’re in danger. Drugs like warfarin, phenytoin, digoxin, and levothyroxine fall into this category. They save lives, but they demand extreme precision. That’s why generic versions of these drugs don’t get approved the same way as ordinary generics. They need bridging studies - specialized tests that prove they behave exactly like the brand-name version in real human bodies.
Why NTI Generics Can’t Follow the Same Rules
Most generic drugs are approved using a simple bioequivalence test. You give a group of healthy volunteers the brand-name drug and the generic, measure how much of the drug enters the bloodstream (Cmax), and how long it stays there (AUC). If the generic’s numbers fall between 80% and 125% of the brand’s, it’s considered equivalent. Simple. Fast. Cheap. But for NTI drugs, that range is too wide. A 20% difference in blood levels could push a patient from safe to toxic - or from effective to ineffective. That’s why regulators like the U.S. FDA and the European Medicines Agency (EMA) tightened the rules. For NTI generics, the acceptable range for Cmax and AUC is now 90.00% to 111.11%. That’s less than half the wiggle room. And it’s not just the numbers - the study design has to be more rigorous too.The Study Design That Makes All the Difference
Standard bioequivalence studies usually use a two-way crossover: half the group gets the brand first, then the generic; the other half gets the generic first, then the brand. For NTI drugs, that’s not enough. The FDA requires a four-way, fully replicated crossover design. That means each participant takes both the brand and the generic twice, in random order. This gives researchers way more data points to spot even the tiniest differences. Why does this matter? Because NTI drugs often have high variability - meaning the same person might absorb the drug differently on different days. A two-way study might miss that. A four-way study catches it. And because of this complexity, these trials need more participants, take longer, and cost more. Studies for NTI generics run $2.5-$3.5 million, compared to $1.5-$2.5 million for standard generics. They also take 12-18 months just for the clinical phase - nearly double the time.What Makes a Drug an NTI Drug?
Not every drug with a small dose range is automatically an NTI drug. The FDA uses five criteria to classify them:- The difference between the lowest effective dose and the lowest toxic dose is no more than 2-fold.
- The range of drug concentrations that work safely is also no more than 2-fold.
- The drug requires regular blood testing to monitor levels.
- Within-subject variability is low to moderate - under 30%.
- Doses are often adjusted in small increments, usually less than 20%.
The Cost of Getting It Right
Developing an NTI generic isn’t just harder - it’s financially risky. Only 6% of generic approvals between 2018 and 2022 were for NTI drugs, even though they make up about 14% of all small-molecule medications. Why? Because the barrier to entry is steep. Manufacturers face three big hurdles:- Cost: The bridging study alone can add $1 million or more to development.
- Time: It takes 3-5 years to bring an NTI generic to market, versus 2-3 for standard ones.
- Expertise: Only 35% of generic drug companies have staff trained in reference-scaled average bioequivalence (RSABE) statistics - the method used to analyze the data.
Who’s in Charge - and How They’re Changing
The FDA and EMA are the main regulators, but they’re not working in isolation. The International Council for Harmonisation (ICH) is trying to align global standards. In 2023, the FDA updated its guidance and added 15 more drugs to the NTI list, bringing the total to 27. That includes common medications like levothyroxine (for thyroid disorders) and cyclosporine (for transplant patients). The EMA’s stance is firm: “NTI drugs require specific bioequivalence study designs that cannot be waived based on product similarity alone.” Even the International Generic and Biosimilar Medicines Association (IGBA), which pushes for faster generic approvals, agrees that NTI drugs are an exception. Waiving bridging studies for these drugs? Not happening. There’s one glimmer of hope: new tools. Physiologically-based pharmacokinetic (PBPK) modeling - computer simulations that predict how a drug behaves in the body - is showing promise. In a 2022 pilot study, PBPK models accurately predicted bioequivalence for warfarin generics without needing full clinical trials. The FDA says it’s “promising,” but still insists: “For the foreseeable future, robust clinical data will remain essential.”
Why This Matters to Patients
You might think: “If it’s generic, it’s the same.” But with NTI drugs, that’s not always true - unless the bridge has been built properly. Switching from brand to generic without proper testing can lead to dangerous outcomes. In one study, patients on levothyroxine who switched to an unverified generic had significant spikes in thyroid-stimulating hormone (TSH) levels - a sign their dose wasn’t right. Some needed hospitalization. Others had to go back to the brand, at triple the cost. The goal isn’t to block generics. It’s to make sure that when a patient switches, they don’t pay with their health. That’s why these bridging studies exist - not to slow things down, but to make sure the switch is safe.The Future: More Access, But Only With Safety
The global market for NTI drugs is worth nearly $80 billion. Generic versions could capture over $30 billion of that - but only if they’re approved correctly. Right now, generic penetration for NTI drugs is just 42%, compared to 85% for non-NTI drugs. That’s a huge gap. Regulators know this. That’s why the FDA launched a pilot program for complex generics, cutting review times by 25% for companies that follow best practices. And by 2025, new ICH guidelines may make it easier to compare NTI drugs across countries, reducing redundant testing. But the core rule won’t change: safety comes first. No shortcuts. No compromises. For drugs where the line between life and danger is thin, the only acceptable standard is precision.What exactly is a narrow therapeutic index (NTI) drug?
An NTI drug is one where the difference between the minimum effective dose and the minimum toxic dose is very small - usually no more than a two-fold difference. Even small changes in how the drug is absorbed can lead to serious side effects or loss of effectiveness. Examples include warfarin, phenytoin, digoxin, and levothyroxine. These drugs often require regular blood monitoring and are dosed in very small increments.
Why can’t NTI generics use the same bioequivalence standards as regular generics?
Regular generics use an 80%-125% range for bioequivalence, but that’s too wide for NTI drugs. A 20% difference in blood levels could cause toxicity or treatment failure. For NTI drugs, regulators require a tighter 90.00%-111.11% range to ensure patient safety. The study design is also more complex - using a four-way crossover instead of two - to account for natural variability in how people absorb these drugs.
How long does it take to develop an NTI generic compared to a regular one?
Developing an NTI generic typically takes 3-5 years, compared to 2-3 years for standard generics. The biggest time sink is the bioequivalence study, which lasts 12-18 months for NTI drugs versus 6-9 months for others. The four-way crossover design requires more participants, longer study periods, and more complex data analysis.
Are there any alternatives to clinical bridging studies for NTI generics?
Yes, researchers are exploring physiologically-based pharmacokinetic (PBPK) modeling - computer simulations that predict how a drug behaves in the body. Early pilot studies, especially with warfarin, show these models can accurately predict bioequivalence. The FDA considers them promising, but still requires clinical data for approval. By 2027, PBPK might reduce the need for full trials for some NTI drugs, but not all.
Why are so few NTI generics on the market?
Only about 6% of generic approvals between 2018 and 2022 were for NTI drugs, despite them making up 14% of small-molecule medications. The main reasons are high costs ($2.5-$3.5 million per study), long development times, lack of in-house expertise, and high rejection rates due to study design flaws. Only 35% of generic manufacturers have staff trained in the complex statistics required for NTI bioequivalence testing.
Do European regulators have the same rules as the FDA for NTI generics?
Yes, the European Medicines Agency (EMA) requires the same level of rigor. In a 2022 position paper, the EMA’s CHMP committee stated that NTI drugs cannot have their bridging study requirements waived based on product similarity alone. Both agencies use similar acceptance criteria (90-111%) and require replicated crossover designs. Harmonization efforts through ICH aim to make these rules consistent worldwide by 2025.
Eliana Botelho
January 30, 2026 AT 02:49Okay but let’s be real - if we’re talking about warfarin and levothyroxine, why are we still letting pharmacies switch generics without telling patients? I had a cousin who went from brand to generic levothyroxine and ended up in the ER with atrial fibrillation because her TSH spiked. No one warned her. No one even asked if she’d switched. The system’s designed to save money, not lives. And don’t give me that ‘it’s bioequivalent’ crap - if it was truly equivalent, why do we need four-way crossovers? Why not just use the same 80-125% rule and call it a day? Because we all know the truth: regulators are terrified of lawsuits, not patients. And the manufacturers? They’re just waiting for someone to slip up so they can undercut everyone else. It’s not science - it’s capitalism with a stethoscope.
Sarah Blevins
January 31, 2026 AT 03:01The statistical rigor required for NTI drug bioequivalence is not merely a regulatory preference - it is a necessary safeguard grounded in pharmacokinetic variability and clinical outcome data. The 90.00–111.11% confidence interval for Cmax and AUC, derived from reference-scaled average bioequivalence (RSABE) methodologies, reduces the risk of therapeutic failure or toxicity by approximately 68% compared to the conventional 80–125% interval. The four-way crossover design further mitigates within-subject variability, which, for drugs like phenytoin, can exceed 25%. Failure to adhere to these standards has been empirically linked to increased hospitalization rates in retrospective cohort studies. The cost and time burdens, while significant, are proportionate to the risk profile.
Kathleen Riley
January 31, 2026 AT 19:32There is a metaphysical tension here, one that mirrors the very nature of therapeutic precision: the human body, a vessel of chaotic biology, is forced into the rigid architecture of regulatory mathematics. We demand that a molecule behave with the consistency of a clock - yet the body, in its ineffable complexity, resists quantification. The NTI drug, then, becomes a symbol of our hubris: we believe that if we measure enough, if we replicate enough, if we standardize enough - we can tame the unpredictable. But what if the difference between life and death isn’t in the AUC, but in the quiet, unmeasured moment when a patient forgets to take their pill? Or when their stress spikes, altering gut permeability? The bridge we build is not between brand and generic - it is between our desire for control and the terrifying truth that biology refuses to be controlled.
Beth Cooper
February 1, 2026 AT 13:03Y’all realize the FDA’s just using NTI as an excuse to keep Big Pharma rich, right? The 90-111% rule? Total scam. I read a whistleblower report once - the same labs that test generics also do contract work for the brand-name companies. Same people, same machines, same data tweaks. And PBPK modeling? That’s just a fancy way of saying ‘we don’t wanna do real trials.’ They’ve been hiding behind ‘safety’ for decades while prices keep climbing. Levothyroxine costs $400 a month in the US? Meanwhile, in Canada, it’s $12. Same pill. Same chemistry. Same everything. But here? We need a $3 million study to prove it’s ‘safe.’ Bullshit. It’s all about monopolies. And don’t even get me started on how the EMA is just following the US like a trained dog. Global collusion, folks. Global collusion.
Donna Fleetwood
February 2, 2026 AT 11:26I just want to say how impressed I am by how seriously we’re taking patient safety here. It’s easy to get frustrated about costs and delays, but honestly? This is one of those rare moments where medicine is doing the right thing, even when it’s hard. I work in a clinic and see patients on these meds every day - one tiny change in their levothyroxine dose and their whole life flips. I’ve seen people lose jobs because their TSH went haywire after a pharmacy switch. So yeah, it takes longer. Yeah, it costs more. But if we can prevent even one hospitalization, it’s worth it. Let’s not rush this. Let’s not let the cheapest option win. Let’s let the safest option win. We owe that to our patients - and to ourselves.
Melissa Cogswell
February 3, 2026 AT 05:35Just a quick note: if you're a generic manufacturer looking at NTI drugs, don't skip the RSABE training. I've seen too many applications get rejected because someone used a standard ANOVA instead of the proper mixed-effects model. The FDA’s guidance documents are actually super clear - they even have R code examples now. Also, consider partnering with a CRO that specializes in NTI studies. The ones with in-house pharmacometricians can save you months. And if you're doing PBPK modeling, make sure your model is validated against actual human PK data - not just in silico simulations. There’s a reason the FDA says ‘robust clinical data remains essential.’ It’s not bureaucracy - it’s science.